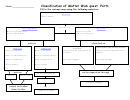
Classification Of Matter Page 4
ADVERTISEMENT
Law of Definite Proportions
• The mass of the compound is equal to the sum of the masses of the elements
that make up the compound.
• The ratio of the mass of each element to the total mass of the compound is a
percentage called the _______________________________________ .
• Equation (fill in):
percent by mass (%) =
x 100
Review Questions
Identify each of the following as an example of a homogeneous mixture or
heterogeneous mixture.
A. a pile of rusty iron filings _______________________
B. 70% isopropyl rubbing alcohol _______________________
C. Saltwater _______________________
D. Gasoline _______________________
Identify each of the following as an example of an element or a compound.!
A. sucrose (table sugar) _______________________
B. the helium in a balloon (He) _______________________
C. baking soda (sodium bicarbonate, NaHCO
) _______________________
3
D. a diamond (carbon, C) _______________________
A 134.50-g sample of aspirin is made up of 6.03 g of hydrogen, 80.70 g of
carbon, and 47.77 g of oxygen. What is the percent by mass of each element in
aspirin?
A 2.89-g sample of sulfur reacts with 5.72 g of copper to form a black
compound. What is the percentage composition of the compound?
Calculate the % mass of each element in C
H
O
(Atomic mass of C = 12, H=1,
6
12
6
O=16)
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4