Classification Of Matter Page 2
ADVERTISEMENT
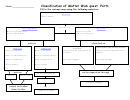
Pure Substances: Elements
• Matter that ______________ be broken down into simpler substances under
normal lab conditions
• Contains only _________ kind of atom
• Draw pictures below of a(n):
o Atom! !
!
Molecule (two or more atoms of the same element)
• Elements (symbols): Na, Au, C
• Where can you find a list of all the elements?
!
_______________________________________________________________
Pure Substances: Compounds
• Can be __________________ into elements
• Composed of two or more elements that combine in a __________________
reaction
• Combine in a __________________ proportion
• Examples: NaCl, H
O, Fe(NO
)
2
3
3
Which are elements and which are compounds?
• Compounds contain more than one element. They always have the same
composition, regardless of source (law of constant composition; law of definite
proportions).
Mixtures
• A blend or combination of two or more __________________
• __________________ chemically combined
• Composition of mixtures is __________________
Mixtures: Heterogeneous
• A __________________ mixture is one that does not blend smoothly
throughout and in which the individual substances remain distinct.
• Mixture with __________________ different parts.
o Examples:
__________________
__________________
__________________
Mixtures: Homogeneous
• A __________________ mixture has constant composition throughout; it
always has a single phase.
• Mixture with _____ __________________ different parts.
o Sea water: __________________
o
Air: __________________
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4