Acids & Bases Calculations Student Notes Page 3
ADVERTISEMENT
Linking pH & pOH
VIII.
A. Higher pH values correspond to lower pOH values
B. Bases have pH > 7 and pOH < 7
C. Acids have pH < 7 and pOH > 7
D. Formula: pH + pOH = 14
–
–4
E. Example #11: Find the pH of an aqueous solution with a [OH
] = 2.20 × 10
M.
–4
F. Example #12: Determine the pH of a 4.3 x 10
M NaOH solution.
More Examples:
IX.
A. Example #13: What is the pH of a solution made by diluting 25 mL of 6.0 M HCl until the final
volume of the solution is 1.75 L? (Hint: M
V
=M
V
)
1
1
2
2
B. Example #14: What is the pH of an aqueous solution of NaOH that contains 5 grams of NaOH
in 2500 mL? (Hint: M = mol /L)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3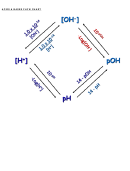 4
4








