Acids & Bases Calculations Student Notes
ADVERTISEMENT
Acids & Bases Calculations Student Notes
pH Scale Values
I.
A. pH scale values range from 0 to 14.
B. Acids:
+
–7
i. pH less than 7
ii. [H
] > 1.0 × 10
M
C. Neutral:
i. pH = 7
ii. [H
+
] = 1.0 × 10
–7
M
D. Bases:
+
–7
i. pH > 7
ii. [H
] < 1.0 × 10
M
Acid/Base Strength
II.
+
A. Strong acids have high [H
] and low pH.
+
B. Strong bases have low [H
] and high pH.
C. At the same molarity, strong acids have lower pH values than weak acids.
D. At the same molarity, strong bases have higher pH values than weak bases.
pH Calculations
III.
+
A. pH is calculated using the equation: pH = – log [H
]
B. Concentration in units of molarity
C. Example #1: What is the pH of a solution with a hydrogen ion concentration of 1.0 × 10
–4
M?
Would this solution be classified as acidic, basic, or neutral?
D. Example #2: If a solution has a [H
+
] concentration of 4.5 x 10
–7
M, what is the pH? Is this an
acidic or basic solution?
[H
+
] Calculations
IV.
+
+
–pH
A. [H
] is calculated using the equation: [H
] = 10
+
B. Example #3: Calculate the [H
] for a solution with a pH of 5.7.
C. Example #4: Calculate the [H
+
] for a solution with a pH of 9.2.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3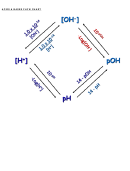 4
4








