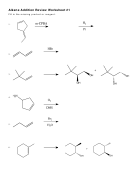
Alkenes Polymerizations Page 2
ADVERTISEMENT
C h e m g u i d e – q u e s t i o n s
4. This question is about PVC - poly(chloroethene). The following is taken from the Chemguide
page:
“
Because of the way the chlorine atoms stick out from the chain at random, and because of their
large size, it is difficult for the chains to lie close together. Poly(chloroethene) is mainly amorphous
with only small areas of crystallinity.”
a) What do you understand by the last sentence “Poly(chloroethene) is mainly amorphous with only
small areas of crystallinity.” ?
b) Despite the lack of crystallinity, PVC is quite hard and rigid. Explain the reason for that.
c) Varying amounts of plasticisers are added to PVC. Briefly, how do they work, and what effect do
they have on the properties of the PVC?
5. a) Write a simple equation to show the formation of poly(tetrafluoroethene) (PTFE) from its
monomer.
b) Describe the properties of PTFE which make it such a useful polymer. (No explanations are
needed.)
(I am not asking for explanations, because what – if anything – you will be expected to say may
well differ depending on your examiners. If PTFE is on your syllabus, it is essential that you look
at past papers and mark schemes to find out exactly what your examiners want.)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2