Chemistry Worksheet
ADVERTISEMENT
Chemistry 151
Lecture Quiz #2
Name
KEY
1/26/06
25 points - 10 minutes
CIN
Multiple choice (Choose BEST answer): 2.5 points each. Place final answers on SCANTRONS. Keep this test for your review.
B
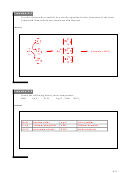
(1) Consider the formation of an ionic compound: 2K(s) + F
(g) ◊ 2 KF. Choose the true statement:
2
a) Potassium (K) has gained an electron.
b) The Fluorine (F) atom has become more negative.
c) F has lost an electron.
d) The product formula unit, KF is not neutral.
-
+
Remember that F gains electrons to form the anion, F
. The metal always loses the electron to form the cation, K
.
C
(2) Consider the following elements: aluminum, fluorine, boron and helium. The order of increasing atomic radii for these elements is:
a) Al, F, He, B
b) He, B, Al, F
c) He, F, B, Al
d) B, He, Al, F
e) F, B, He, Al
Remember the atomic radii trends: toward the left and down the periodic table.
A
(3) Consider the following elements: aluminum, fluorine, boron and helium. The order of decreasing metallic character for these elements is:
a) Al, B, F, He
b) He, B, Al, F
c) Al, F, B, He
d) B, He, Al, F
Remember that the more to the left and down you are in the periodic table, the more metallic the element.
D
(4) How many dots are present in the correct Lewis dot structure for silicon (Si)?
a) 1
b) 2
c) 3
d) 4
e) 5
2
2
1
2
1
Remember that the electronic configuration of B is 1s
2s
2p
; there are 3 valence electrons (i.e. 2s
2p
). So 3 dots.
B
(5) What change in the number of valence electrons does the oxygen atom tend to have according to the octet rule?
a) It loses 1 electron, b) it gains 2 electrons, c) it loses 2 electrons, d) it gains 3 electrons.
Oxygen is group 6. To achieve noble gas configuration (group 8A), it needs 2 more electrons.
D
(6) Suppose that X is a group 2A element and Y is a group 5A element. What is the correct formula for the ionic compound composed of X and Y?
a) XY
b) X
Y
c) X
Y
d) X
Y
e) XY
2
5
2
3
3
2
2
2+
3-
2+
3-
X must be X
and Y
. The only way to combine them and get neutrality is three X
‘s for every two Y
‘s. So, X
Y
.
3
2
C
(7) Which of the following statements about ionic compounds is incorrect?
a) In forming ionic compounds, nonmetallic elements gain electrons
b) Ionic compounds tend to form crystalline structures
c) Ionic compounds can be formed between two metal ions.
d) Some ionic compounds are water soluble and soft.
Metal ions are positive. You can’t have 2 positive ions (“cations”) combining to form a neutral ionic compound. Ionic compounds are made up of cations and
anions.
D
(8) Consider the following electronegativities: H = 2.2, Li =1.0, B =2.0, C= 2.5, O =3.5.
Choose the correct statement below:
a) Boron hydride is an ionic compound.
b) Lithium hydride is an ionic compound.
c) Methane (CH4) is an ionic compound.
d) Lithium oxide is an ionic compound.
The difference in electronegativities are all inconsistent with the statement except for d). for Li
O, ∆eneg = 3.5-1.0=2.5. The rule of thumb is if ∆eneg is greater
2
than 1.9, it is an ionic compound.
D
(9) Which of the following is either mismatched or misnamed?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2