Chemistry Worksheets Page 3
ADVERTISEMENT
2. Balance the following half-reactions for both atoms and electrons by adding the appropriate number of
electrons to the correct side of the equation. Also identify each as either an oxidation or reduction.
2+
2+
-
→ Pb
→ Pb
a. Pb
Pb
+ 2e
reduction
-
–
-
→ Cl
→ 2 Cl
b. Cl
Cl
+ 2 e
reduction
2
2
3+
2+
3+
–
2+
→ Fe
→ Fe
c. Fe
Fe
+ e
reduction
+
+
–
O → NO + H
O → 2 NO + 2 H
d. N
O + H
N
O + H
+ 2e
oxidation
2
2
2
2
3. Break each equation into two half-reactions. Identify each half-reaction as oxidation or reduction.
+
2+
→ Cu
a. Cu + 2 H
+ H
2
2+
–
Cu → Cu
+ 2 e
oxidation
+
–
→ H
2 H
+ 2 e
reduction
2
b. 2 Al + 3 S → Al
S
2
3
+3
–
2 Al → 2Al
+ 6 e
oxidation
–
2-
→ 3 S
3S + 6e
reduction
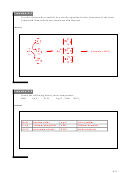
4. Balance the following equations using the half-reaction method.
→ NaBr
a. Na + Br
2
Step 2
Step 1
Step 3
Write the two balanced half-
Balance for
Add the half-reactions, replacing any
reactions, removing any
spectator ions that were removed and/or
electrons
spectator ions:
recombining compounds
+
–
+
–
Na → Na
2 Na → 2 Na
multiply by 2
+ e
+ 2e
-
–
-
–
→ 2 Br
→ 2 Br
Br
+ 2 e
Br
+ 2 e
2
2
+
–
→ 2 Na
added together:
2 Na + Br
+ 2 Br
2
→ 2 NaBr
2 Na + Br
reform compound:
2
Unit 6: Redox & Electrochemistry
Practice Set 3
Page 3 of 4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4