Chemistry Eoc Assessment Reference Materials
ADVERTISEMENT
Chemistry EOC Assessment
Reference Materials
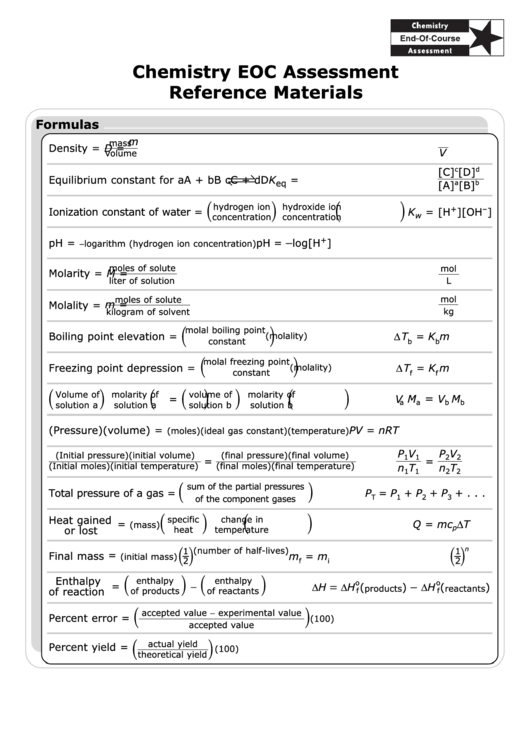
Formulas
m
mass
Density =
D =
V
volume
c
d
[C]
[D]
Equilibrium constant for aA + bB
cC + dD
K
=
eq
a
b
[A]
[B]
(
)(
)
hydrogen ion
hydroxide ion
+
–
Ionization constant of water =
K
= [H
][OH
]
w
concentration
concentration
−
+
−logarithm (hydrogen ion concentration)
pH =
pH =
log[H
]
moles of solute
mol
Molarity =
M =
liter of solution
L
mol
moles of solute
m =
Molality =
kg
kilogram of solvent
(
molal boiling point
)
ΔT
Boiling point elevation =
(molality)
= K
m
constant
b
b
(
)
molal freezing point
ΔT
Freezing point depression =
(molality)
= K
m
constant
f
f
(
)(
)
(
)(
)
Volume of
molarity of
volume of
molarity of
=
=
V
M
V
M
a
a
b
b
solution a
solution a
solution b
solution b
(Pressure)(volume) =
PV = nRT
(moles)(ideal gas constant)(temperature)
P
V
P
V
(Initial pressure)(initial volume)
(final pressure)(final volume)
1
1
2
2
=
=
(Initial moles)(initial temperature)
(final moles)(final temperature)
n
T
n
T
1
1
2
2
(
)
sum of the partial pressures
Total pressure of a gas =
P
= P
+ P
+ P
+ . . .
of the component gases
T
1
2
3
(
)(
)
Heat gained
specific
change in
ΔT
=
Q = mc
(mass)
p
or lost
heat
temperature
n
( )
(number of half-lives)
( )
1
1
Final mass =
m
= m
(initial mass)
2
2
f
i
(
)
(
)
Enthalpy
enthalpy
enthalpy
=
−
−
ΔH
ΔH (
o
ΔH (
o
=
)
)
products
reactants
of reaction
of products
of reactants
f
f
accepted value − experimental value
(
)
Percent error =
(100)
accepted value
(
)
actual yield
Percent yield =
(100)
theoretical yield
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4








