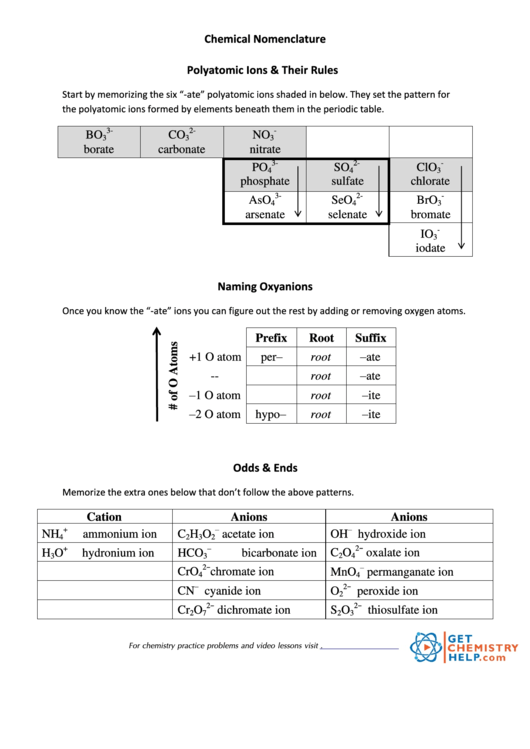
Polyatomic Ions Reference Sheet
ADVERTISEMENT
Chemical Nomenclature
Polyatomic Ions & Their Rules
Start by memorizing the six “-ate” polyatomic ions shaded in below. They set the pattern for
the polyatomic ions formed by elements beneath them in the periodic table.
3-
2-
-
BO
CO
NO
3
3
3
borate
carbonate
nitrate
3-
2-
-
PO
SO
ClO
4
4
3
phosphate
sulfate
chlorate
3-
2-
-
AsO
SeO
BrO
4
4
3
arsenate
selenate
bromate
-
IO
3
iodate
Naming Oxyanions
Once you know the “-ate” ions you can figure out the rest by adding or removing oxygen atoms.
Prefix
Root
Suffix
–ate
+1 O atom
per–
root
–ate
--
root
–1 O atom
–ite
root
–2 O atom
–ite
hypo–
root
Odds & Ends
Memorize the extra ones below that don’t follow the above patterns.
Cation
Anions
Anions
–
–
+
NH
ammonium ion
C
H
O
acetate ion
OH
hydroxide ion
4
2
3
2
–
+
2−
H
O
hydronium ion
HCO
bicarbonate ion
C
O
oxalate ion
3
3
2
4
–
2−
CrO
chromate ion
MnO
permanganate ion
4
4
–
2−
CN
cyanide ion
O
peroxide ion
2
2−
2−
Cr
O
dichromate ion
S
O
thiosulfate ion
2
7
2
3
For chemistry practice problems and video lessons visit .
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








