Atoms And Valence Electrons Reference Sheet
ADVERTISEMENT
Atoms and Valence Electrons Reference Sheet
Valence Electrons: those electrons that have the highest energy level and are held most loosely.
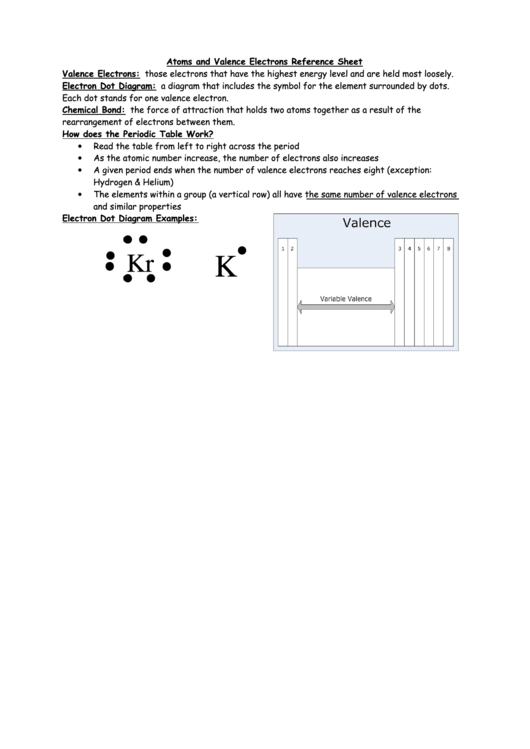
Electron Dot Diagram: a diagram that includes the symbol for the element surrounded by dots.
Each dot stands for one valence electron.
Chemical Bond: the force of attraction that holds two atoms together as a result of the
rearrangement of electrons between them.
How does the Periodic Table Work?
Read the table from left to right across the period
•
As the atomic number increase, the number of electrons also increases
•
A given period ends when the number of valence electrons reaches eight (exception:
•
Hydrogen & Helium)
The elements within a group (a vertical row) all have the same number of valence electrons
•
and similar properties
Electron Dot Diagram Examples:
Kr
K
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








