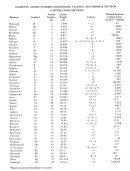
Atomic Chart- Elements Practice Page 3
ADVERTISEMENT
UNIT 3 - ATOMS
4.)
7.4 x 10
molecules of KCl
24
5.)
3.5 x 10
25
molecules of CuSO
4
Part 2 - Determine the number of molecules in each of the quantities below.
6.)
2.5 moles of NaCl
7.)
0.50 moles of H
SO
2
4
8.)
1.70 moles of KMnO
4
9.)
0.25 moles of KCl
10.)
3.2 moles of CuSO
4
MOLES AND MASS
Part 1 - Determine the number of moles in each of the quantities below.
1.)
25.0 grams of NaCl
2.)
125 grams of H
SO
2
4
3.)
100. grams of KMnO
4
4.)
74.5 grams of KCl
5.)
35 grams of CuSO
4
Part 2 - Determine the mass (number of grams) in each of the quantities below.
6.)
2.5 moles of NaCl
7.)
0.50 moles of H
SO
2
4
8.)
1.70 moles of KMnO
4
9.)
0.25 moles of KCl
10.)
3.2 moles of CuSO
4
PRACTICE WITH MOLE CONVERSIONS
1. How many moles are equal to 2.548 grams of boron trifluoride, BF
?
3
2. How many grams are there in 2.45 x 10
molecules of ammonia, NH
?
24
3
3. How many moles are equal to 5.29 x 10
23
atoms of carbon?
4. What is the mass (in grams) of 6.759 moles of sodium chloride, NaCl?
5. How many molecules of H
PO
are contained in 0.257 moles of H
PO
?
3
4
3
4
6. How many atoms of copper are equal to 49.5 grams of copper?
7. What is the mass (in grams) of 7.14 x 10
23
molecules of C
H
O
?
6
12
6
8. How many moles are equal to 8.392 x 10
23
atoms of uranium?
9. What is the mass (in grams) of 5.685 moles of sodium bicarbonate, NaHCO
?
3
10. How many molecules are equal to 0.027 moles of calcium carbonate, CaCO
?
3
11. How many moles are equal to 93.75 grams of sodium sulfate, Na
SO
?
2
4
12. How many molecules are equal to 103.74 grams of lead nitrate, Pb(NO
)
?
3
2
*13. A large piece of aluminum foil has a mass of 35.25 grams. What mass of pure tin would contain
the same number of atoms as the aluminum foil?
3
Practice - Honors
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3