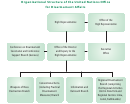
Structure Of The Atom Page 3
ADVERTISEMENT
CHEMISTRY
Electron shells
The electrons in an atom are arranged in
electron
shells, or
energy levels
around the nucleus.
Filling the shells
Electronic configurations
You can work out the
electronic
The diagram shows the electronic
con guration
of an element from its
con guration of sodium. This can also
atomic number. The electrons ll the
be written in numbers: 2.8.1
shells that are closest to the nucleus
symbol for the
rst.
element
First (inner) shell - 2 electrons
electrons are shown
Na
using dots or crosses
Second shell - 8 electrons
circles represent
Third shell - 8 electrons
electron shells
The atomic number of potassium is 19. Explain how its electrons
are arranged.
An atom has the same number of protons and
electrons, so a potassium atom has 19 electrons.
The inner shell lls up rst, and this shell is full
when it has 2 electrons. The second shell has
8 electrons, and this is also full. This leaves 9
electrons. The third shell can hold 8 electrons, so
that is full as well. The last shell has 1 electron in it.
We write this as 2.8.8.1.
Electrons and groups
1
2
3
4
5
6
7
0
The group numbers are the same as the
number of electrons in the outer shells
H
(apartfrom group 0, where the outer
1. Draw diagrams to show the
1.
He
target
shells are full).
electron arrangements in
D-C
lithium and oxygen. (4 marks)
Li
Be
B
C
N
O
F
Ne
2. Sulfur is in group 6 of the
2.
2. 2.
target
periodic table. Explain how you
G-E
Na
Mg
Al
Si
P
S
Cl
Ar
can work out the number of
outer electrons in a sulfur atom
The number of occupied shells is the same
without knowing its atomic
K
Ca
as the period number.
number.
(2 marks)
39
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4