Structure Of The Atom
ADVERTISEMENT
CHEMISTRY
Structure of the atom
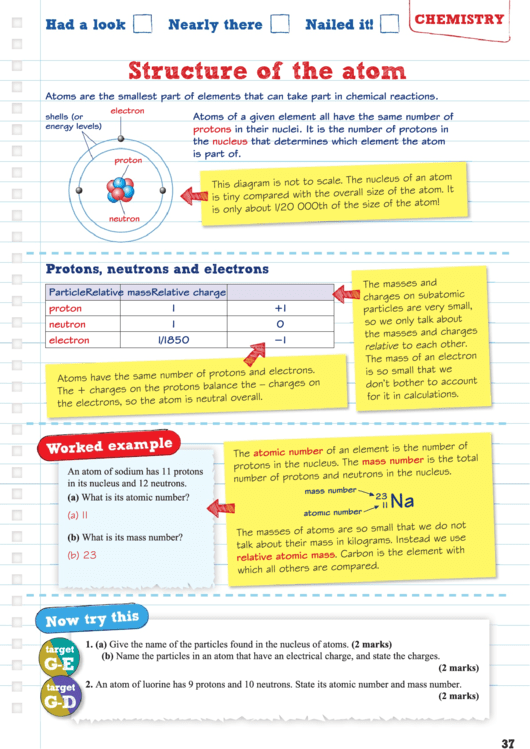
Atoms are the smallest part of elements that can take part in chemical reactions.
electron
Atoms of a given element all have the same number of
shells (or
energy levels)
protons
in their nuclei. It is the number of protons in
the
nucleus
that determines which element the atom
is part of.
proton
neutron
Protons, neutrons and electrons
Particle
Relative mass
Relative charge
proton
1
+1
neutron
1
0
electron
1/1850
−1
An atom of sodium has 11 protons
in its nucleus and 12 neutrons.
(a) What is its atomic number?
(a) 11
(b) What is its mass number?
(b) 23
1. (a) Give the name of the particles found in the nucleus of atoms.
(2 marks)
target
(b) Name the particles in an atom that have an electrical charge, and state the charges.
G-E
(2 marks)
2. An atom of luorine has 9 protons and 10 neutrons. State its atomic number and mass number.
2.
target
(2 marks)
G-D
37
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4