Mole Conversions Worksheet Page 2
ADVERTISEMENT
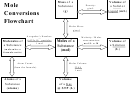
2. Find the mass in grams of 2.023 moles of F 2 .
3. Determine the volume in liters occupied by 14 moles of nitrogen gas at STP.
25
4. Find the number moles in 8.056x10
molecules of chlorine gas.
6. Aspartame is an artificial sweetener that is 160 times sweeter than sucrose (table sugar) when dissolved in
water. It is marketed by G.D. Searle as Nutra Sweet. The molecular formula of aspartame is C 14 H 18 N 2 O 5 .
a) Calculate the molar mass of aspartame.
b) How many moles of molecules are in 10 g of aspartame?
c) What is the mass in grams of 1.56 moles of aspartame?
d) How many molecules are in 5 mg of aspartame? (hint: multiple step problem )***
e) How many atoms of nitrogen are in 1.2 grams of aspartame? (hint: multiple step problem )***
**Last 2 problems are “more” challenging; do your best on letter ‘e’ – we have not gone over it yet **
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2