Mole Conversions Worksheet
ADVERTISEMENT
Mole Conversions Practice
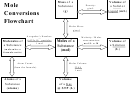
Moles
Grams
Moles
Particles
There are three mole equalities. They are:
23
1 mol = 6.02 x 10
particles (atoms, molecules, formula units)
1 mol = molar mass/formula mass (periodic table)
1 mol = 22.4 L for a gas at STP
We will work with ALL of the equalities today!
Each equality can be written as a set of two conversion factors (the things you write in your T-Chart)
Mole-Volume Conversions
1. Determine the volume, in liters, occupied by 0.030 moles of a gas at STP.
2. How many moles of argon atoms are present in 11.2 L of argon gas at STP?
3. What is the volume of 0.05 mol of neon gas at STP?
4. What is the volume of 1.2 moles of water vapor at STP?
Mixed Mole Conversions
1. How many moles are in 83.36 L of oxygen gas at STP?
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2