Solubility Chart
ADVERTISEMENT
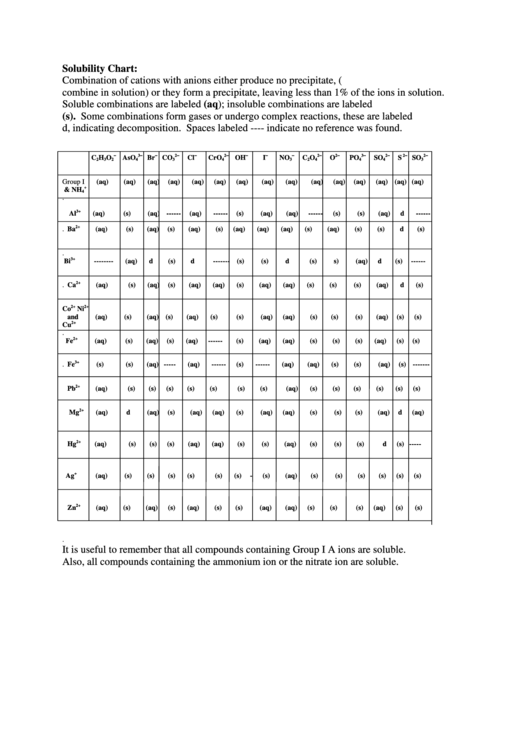
Solubility Chart:
Combination of cations with anions either produce no precipitate, (i.e. the ions do not
combine in solution) or they form a precipitate, leaving less than 1% of the ions in solution.
Soluble combinations are labeled (aq); insoluble combinations are labeled
(s). Some combinations form gases or undergo complex reactions, these are labeled
d, indicating decomposition. Spaces labeled ---- indicate no reference was found.
3
2
2
2
2
3
2
2
2
C
H
O
¯ AsO
¯ Br¯ CO
¯ Cl¯
CrO
¯ OH¯
I¯
NO
¯ C
O
¯ O
¯
PO
¯ SO
¯ S
¯ SO
¯
2
3
2
4
3
4
3
2
4
4
4
3
Group I
(aq)
(aq)
(aq)
(aq)
(aq)
(aq)
(aq)
(aq)
(aq)
(aq)
(aq)
(aq)
(aq) (aq) (aq)
+
& NH
4
.
3+
Al
(aq)
(s)
(aq) ------
(aq)
------
(s)
(aq)
(aq)
------
(s)
(s)
(aq)
d
------
2+
. Ba
(aq)
(s)
(aq)
(s)
(aq)
(s)
(aq)
(aq)
(aq)
(s)
(aq)
(s)
(s)
d
(s)
.
3+
Bi
--------
(aq)
d
(s)
d
------- (s)
(s)
d
(s)
s)
(aq)
d
(s)
------
2+
. Ca
(aq)
(s)
(aq)
(s)
(aq)
(aq)
(s)
(aq)
(aq)
(s)
(s)
(s)
(aq)
d
(s)
2+
2+
Co
Ni
and
(aq)
(s)
(aq) (s)
(aq)
(s)
(s)
(aq)
(aq)
(s)
(s)
(s)
(aq)
(s)
(s)
2+
Cu
.
2+
Fe
(aq)
(s)
(aq)
(s)
(aq)
------
(s)
(aq)
(aq)
(s)
(s)
(s)
(aq)
(s)
(s)
3+
. Fe
(s)
(s)
(aq) -----
(aq)
------
(s)
------
(aq)
(aq)
(s)
(s)
(aq)
(s) -------
2+
Pb
(aq)
(s)
(s)
(s)
(s)
(s)
(s)
(s)
(aq)
(s)
(s)
(s)
(s)
(s)
(s)
2+
Mg
(aq)
d
(aq)
(s)
(aq)
(aq)
(s)
(aq)
(aq)
(s)
(s)
(s)
(aq)
d
(aq)
2+
Hg
(aq)
(s)
(s)
(s)
(aq)
(aq)
(s)
(s)
(aq)
(s)
(s)
(s)
d
(s) -----
+
Ag
(aq)
(s)
(s)
(s)
(s)
(s)
(s)
-
(s)
(aq)
(s)
(s)
(s)
(s)
(s)
(s)
2+
Zn
(aq)
(s)
(aq)
(s)
(aq)
(s)
(s)
(aq)
(aq)
(s)
(s)
(s)
(aq)
(s)
(s)
.
It is useful to remember that all compounds containing Group I A ions are soluble.
Also, all compounds containing the ammonium ion or the nitrate ion are soluble.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1