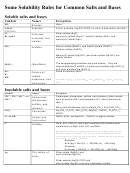
Solubility Rules For Ionic Compounds
ADVERTISEMENT
Solubility Rules for Ionic Compounds
The rules are meant as a guide only. There are exceptions to these rules.
1. Salts of the alkali metals are soluble. (Note: The alkali metals are in group 1.)
e.g.
If M = Li, Na or K, then MX, M
X, M
X, etc. are soluble regardless of what X is.
2
3
+
2. Ammonium (
NH ) salts are soluble.
4
e.g.
NH X, (
NH )
X, (
NH )
X, etc. are soluble regardless of what X is.
2
3
4
4
4
− −
3. Nitrates
NO
are soluble.
3
e.g.
MNO
, M(NO
)
, M(NO
)
, etc. are soluble regardless of what M is.
3
3
2
3
3
− −
− −
− −
4. Halides i.e. chlorides (Cl
), bromides (Br
) and iodides (I
) are soluble except for the
2+
+
+
2+
halides of lead (Pb
), mercury (Hg
and
Hg ) and silver (Ag
).
2
e.g.
If X = Cl, Br or I, then MX, MX
, MX
, etc. are soluble unless M = Pb, Hg or Ag.
2
3
−
2
5. Sulfates (
SO ) are soluble except for the sulfates of calcium, strontium, barium, silver
4
mercury and lead.
e.g.
M
SO
, MSO
, M
(SO
)
, etc. are soluble unless M is from group 2 (the alkaline
2
4
4
2
4
3
earths) or M = Pb, Hg or Ag.
2− −
−
−
2
3
6. Carbonates (
CO ), phosphates (
PO ) and sulfides (S
) are insoluble except for
3
4
(i) the carbonates/phosphates/sulfides of the alkalis (because of Rule 1), and
(ii) ammonium carbonate/phosphate/sulfide (because of Rule 2).
− −
7. Hydroxides (OH
) are insoluble or slightly soluble except for the hydroxides of the
alkalis (because of Rule 1).
Note: The hydroxides of group 2 (the alkaline earth metals) are slightly soluble. Virtually
all other hydroxides are insoluble.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1