Solubility Rules For Ionic Compounds Chart
ADVERTISEMENT
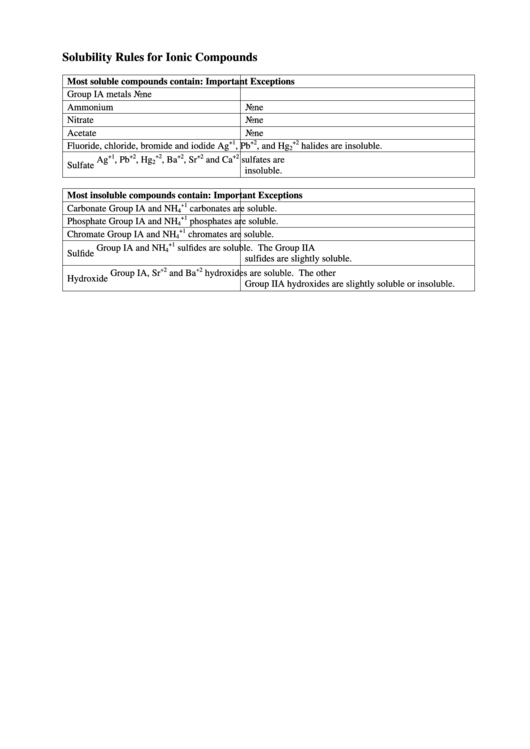
Solubility Rules for Ionic Compounds
Most soluble compounds contain:
Important Exceptions
Group IA metals
None
Ammonium
None
Nitrate
None
Acetate
None
+1
+2
+2
Fluoride, chloride, bromide and iodide
Ag
, Pb
, and Hg
halides are insoluble.
2
+1
+2
+2
+2
+2
+2
Ag
, Pb
, Hg
, Ba
, Sr
and Ca
sulfates are
2
Sulfate
insoluble.
Most insoluble compounds contain:
Important Exceptions
+1
Carbonate
Group IA and NH
carbonates are soluble.
4
+1
Phosphate
Group IA and NH
phosphates are soluble.
4
+1
Chromate
Group IA and NH
chromates are soluble.
4
+1
Group IA and NH
sulfides are soluble. The Group IIA
4
Sulfide
sulfides are slightly soluble.
+2
+2
Group IA, Sr
and Ba
hydroxides are soluble. The other
Hydroxide
Group IIA hydroxides are slightly soluble or insoluble.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1