Solubility Rules For Inorganic Salts
ADVERTISEMENT
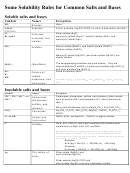
SOLUBILITY RULES FOR INORGANIC SALTS
The following is a brief list of guidelines with some of the major exceptions noted.
Soluble Salts
+
+
+
+
+
+
1.
All group IA (Na
, K
, Li
, Rb
, Cs
) salts and all NH
salts are soluble.
4
2.
All nitrates, acetates and perchlorates are soluble (except AgC
H
O
, KClO
).
2
3
2
4
+
2+
2+
3.
All halides are soluble (except those of Ag
, Hg
, Pb
).
2
4.
All sulfates are soluble (except BaSO
, PbSO
, SrSO
).
4
4
4
Insoluble Salts and Metal Hydroxides
+
5.
All metal hydroxides are insoluble (except those of groups IA, IIA, and NH
, but
4
Mg(OH)
is insoluble).
2
+
6.
All carbonates are insoluble except those of Group IA and NH
.
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1