Common Polyatomic Ions List
ADVERTISEMENT
Student
# :
________
Common
P olyatomic
I ons
L ist
Block:
________
Fall
2 012
C hemistry
I
–
M r.
P atel
Name
_ __________________________________________________
D ate
_ _____________________
Each
s et
o f
i ons
w ill
b e
a ssigned
a t
d ifferent
t imes.
O nce
a
s et
o f
i ons
h as
b een
a ssigned,
s tudents
s hould
become
v ery
f amiliar
w ith
t he
n ame
a nd
c orresponding
s ymbol.
T his
i nformation
w ill
b e
f air
g ame
f or
any
a ssessments
a t
a ny
t ime
t hrough
t he
c ourse.
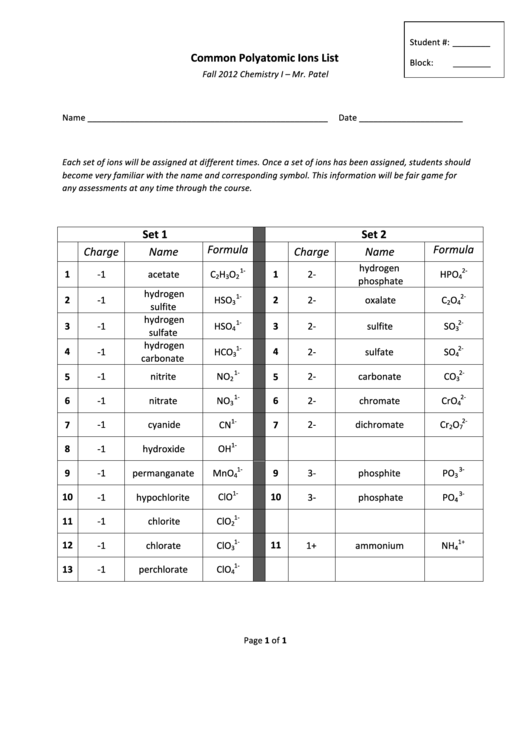
Set
1
Set
2
Formula
Formula
Charge
Name
Charge
Name
hydrogen
1-‐
2-‐
1
-‐1
acetate
C
H
O
1
2-‐
HPO
2
3
2
4
phosphate
hydrogen
1-‐
2-‐
2
-‐1
HSO
2
2-‐
oxalate
C
O
3
2
4
sulfite
hydrogen
1-‐
2-‐
3
-‐1
HSO
3
2-‐
sulfite
SO
4
3
sulfate
hydrogen
1-‐
2-‐
4
-‐1
HCO
4
2-‐
sulfate
SO
3
4
carbonate
1-‐
2-‐
5
-‐1
nitrite
NO
5
2-‐
carbonate
CO
2
3
1-‐
2-‐
6
-‐1
nitrate
NO
6
2-‐
chromate
CrO
3
4
1-‐
2-‐
7
-‐1
cyanide
CN
7
2-‐
dichromate
Cr
O
2
7
1-‐
8
-‐1
hydroxide
OH
1-‐
3-‐
9
-‐1
permanganate
MnO
9
3-‐
phosphite
PO
4
3
1-‐
3-‐
10
-‐1
hypochlorite
ClO
10
3-‐
phosphate
PO
4
1-‐
11
-‐1
chlorite
ClO
2
1-‐
1+
12
-‐1
chlorate
ClO
11
1+
ammonium
NH
3
4
1-‐
13
-‐1
perchlorate
ClO
4
Page
1
o f
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








