Chemistry Flashcards Template Page 6
ADVERTISEMENT
Rules
Old School Lewis Dots
1. Sum all valence electrons, including
charges
You Try:
2. Single Bonds
HCN
3. Outer atoms get an octet except H
N
4. Center gets rest even if it violates the octet
2
5. Double/triple bonds if center atom still
AlN
does not have an octet
O
2
You try:
Lewis Dots
SF
4
KrF
4
NH
3
Cl
O
2
NCl
3
ClF
3
SF
6
H
SO
2
4
Br
O
2
ClF
5
Warm-Up:
Lewis Dots
CH
CH
NH
3
2
2
SeF
CO
4
2
KrCl
HCN
4
H
O
-
CN
2
2
BaCl
(this is an ionic cmpd)
2
-
ICl
4
+
NO
6
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Life
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17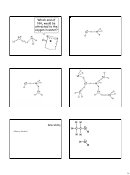 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27








