Solutions - Dilutions Concentration Worksheet With Answers
ADVERTISEMENT
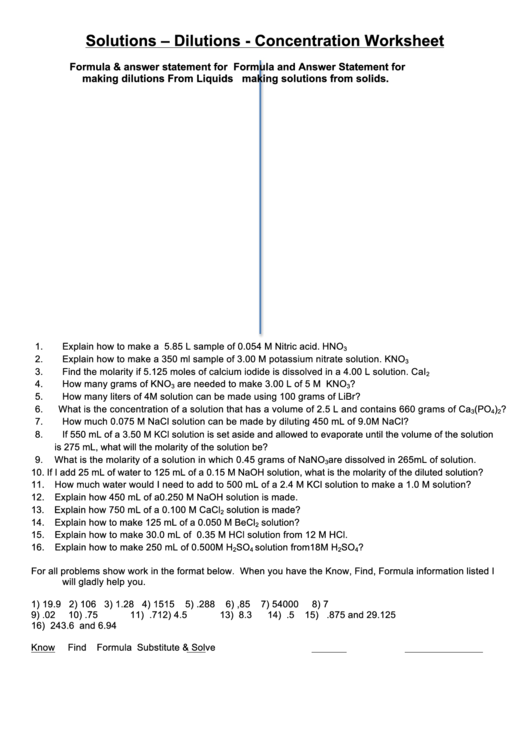
Solutions – Dilutions - Concentration Worksheet
Formula & answer statement for
Formula and Answer Statement for
making dilutions From Liquids
making solutions from solids.
1.
Explain how to make a 5.85 L sample of 0.054 M Nitric acid. HNO
3
2.
Explain how to make a 350 ml sample of 3.00 M potassium nitrate solution. KNO
3
3.
Find the molarity if 5.125 moles of calcium iodide is dissolved in a 4.00 L solution. CaI
2
4.
How many grams of KNO
are needed to make 3.00 L of 5 M KNO
?
3
3
5.
How many liters of 4M solution can be made using 100 grams of LiBr?
6.
What is the concentration of a solution that has a volume of 2.5 L and contains 660 grams of Ca
(PO
)
?
3
4
2
7.
How much 0.075 M NaCl solution can be made by diluting 450 mL of 9.0M NaCl?
8.
If 550 mL of a 3.50 M KCl solution is set aside and allowed to evaporate until the volume of the solution
is 275 mL, what will the molarity of the solution be?
9.
What is the molarity of a solution in which 0.45 grams of NaNO
are dissolved in 265mL of solution.
3
10.
If I add 25 mL of water to 125 mL of a 0.15 M NaOH solution, what is the molarity of the diluted solution?
11.
How much water would I need to add to 500 mL of a 2.4 M KCl solution to make a 1.0 M solution?
12.
Explain how 450 mL of a 0.250 M NaOH solution is made.
13.
Explain how 750 mL of a 0.100 M CaCl
solution is made?
2
14.
Explain how to make 125 mL of a 0.050 M BeCl
solution?
2
15.
Explain how to make 30.0 mL of 0.35 M HCl solution from 12 M HCl.
16.
Explain how to make 250 mL of 0.500M H
SO
solution from18M H
SO
?
2
4
2
4
For all problems show work in the format below. When you have the Know, Find, Formula information listed I
will gladly help you.
1) 19.9
2) 106
3) 1.28
4) 1515
5) .288
6) ,85
7) 54000
8) 7
9) .02
10) .75
11) .7
12) 4.5
13) 8.3
14) .5
15) .875 and 29.125
16) 243.6 and 6.94
Know
Find
Formula
Substitute & Solve
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1








