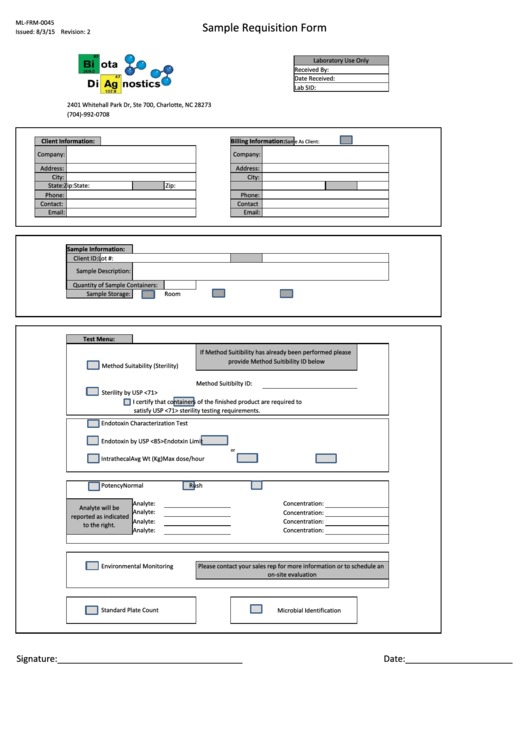
Form Ml-Frm-0045 - Sample Requisition Form
ADVERTISEMENT
ML-FRM-0045
Sample Requisition Form
Issued: 8/3/15 Revision: 2
Laboratory Use Only
Received By:
Date Received:
Lab SID:
2401 Whitehall Park Dr, Ste 700, Charlotte, NC 28273
(704)-992-0708
Client Information:
Billing Information:
Same As Client:
Company:
Company:
Address:
Address:
City:
City:
State:
Zip:
State:
Zip:
Phone:
Phone:
Contact:
Contact
Email:
Email:
Sample Information:
Client ID:
Lot #:
Sample Description:
Quantity of Sample Containers:
Sample Storage:
Room Temp.
Refrigerated
Frozen
Test Menu:
If Method Suitibility has already been performed please
provide Method Suitibility ID below
Method Suitability (Sterility)
Method Suitibilty ID:
Sterility by USP <71>
I certify that
containers of the finished product are required to
satisfy USP <71> sterility testing requirements.
Endotoxin Characterization Test
Endotoxin by USP <85>
Endotxin Limit
or
Intrathecal
Avg Wt (Kg)
Max dose/hour
Potency
Normal
Rush
Analyte:
Concentration:
Analyte will be
Analyte:
Concentration:
reported as indicated
Analyte:
Concentration:
to the right.
Analyte:
Concentration:
Environmental Monitoring
Please contact your sales rep for more information or to schedule an
on-site evaluation
Standard Plate Count
Microbial Identification
Signature:______________________________________
Date:______________________
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1








