Chemistry Reference Materials Page 2
ADVERTISEMENT
STAAR CHEMISTRY
REFERENCE MATERIALS
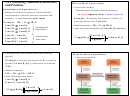
OTHER FORMULAS
mass
m
Density
D
=
=
volume
V
(
)
accepted value
−
experimental value
=
(100)
Percent error
accepted value
(
)
actual yield
=
(100)
Percent yield
theoretical yield
CONSTANTS AND CONVERSIONS
23
Avogadro
’s number =
6.02
10
particles per mole
×
−
34
h
Planck’s constant =
6.63
10
J ⋅ s
=
×
m
8
c =
speed of light
=
3.00
×
10
s
(
)
2
mol
−
14
K
ionization constant of water
1.00
10
=
=
×
w
L
0
4
1
beta particle ( )
β =
e
neutron
n
alpha particle ( )
α =
He
=
0
2
−1
standard temperature and pressure (STP) = 0°C and 1 atm
0°C = 273 K
L
volume of ideal gas at STP
=
22.4
mol
3
1 cm
=
1 mL
=
1 cc
1 atm = 760 mm Hg = 101.3 kPa
L
atm
L
kPa
L
mm Hg
⋅
⋅ ⋅
⋅
R =
ideal gas constant
0.0821
=
8.31
62.4
=
=
mol K
mol K
mol K
⋅
⋅
⋅
1 calorie (cal) = 4.18 joules (J)
1000 calories (cal) = 1 Calorie (Cal) = 1 kilocalorie (kcal)
RULES FOR SIGNIFICANT FIGURES
1. Non-zero digits and zeros between non-zero digits are always significant.
2. Leading zeros are not significant.
3. Zeros to the right of all non-zero digits are only significant if a decimal point is shown.
4. For values written in scientific notation, the digits in the coefficient are significant.
5. In a common logarithm, there are as many digits after the decimal point as there are
significant figures in the original number.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4