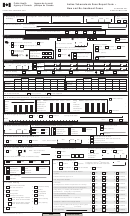
Invasive Methicillin-Resistant-Staphylococcus Aureus Active Bacterial Core Surveillance (Abcs) Case Report Form - 2015
ADVERTISEMENT
Patient ID: _____ _____ _____ _____ _____ _____ _____ _____
– ACTIVE BACTERIAL CORE SURVEILLANCE CASE REPORT –
Phone No.: (
)
Patient's Name:
(Last, First, M.I.)
Patient
Address:
Chart No
.:
(Number, Street, Apt. No.)
Hospital:
(City, State)
(Zip Code)
– Patient identifier information is NOT transmitted to CDC –
DEPARTMENT OF
INVASIVE METHICILLIN-RESISTANT • STAPHYLOCOCCUS AUREUS
HEALTH & HUMAN SERVICES
CENTERS FOR DISEASE CONTROL
A
B
C
CTIVE
ACTERIAL
ORE SURVEILLANCE (ABCs) CASE REPORT – 2015
AND PREVENTION
ATLANTA, GA 30329
– SHADED AREAS BELOW INDICATE CORE VARIABLES –
Form Approved OMB No. 0920-0978
1. STATE:
2. COUNTY:
3. STATE I.D.:
4a. HOSPITAL/LAB I.D. WHERE
4b. HOSPITAL I.D. WHERE PATIENT TREATED:
(Residence of patient)
(Residence of Patient)
CULTURE IDENTIFIED:
6. DATE OF BIRTH:
5. SEX:
8. STERILE SITE(S) FROM WHICH MRSA WAS INITIALLY
7a. AGE:
ISOLATED: (Check all that apply)
M
1
ale
1
Blood
1
Pericardial fluid
1
Internal body site (specify)
Mo.
Day
Year
2
Female
_____________________
1
CSF
1
Joint/Synovial fluid
7b. Is age in day/mo/yr?
1
Bone
1
Pleural fluid
1
Other sterile site (specify)
1
Peritoneal fluid
1
Muscle
1
Days 2
Mos. 3
Yrs.
______________________
9. DATE OF INITIAL CULTURE:
10. WAS THE PATIENT HOSPITALIZED AT THE TIME OF,
11. WAS CULTURE COLLECTED >3 CALENDAR DAYS
OR WITHIN 30 CALENDAR DAYS AFTER, INITIAL CULTURE?
AFTER HOSPITAL ADMISSION?
Mo.
Day
Year
1
Yes (HO-MRSA case) 2
No (Complete CRF, CA-MRSA or HACO-MRSA case)
1
Yes
2
No
9
Unknown
If YES: Date of admission
If yes, was the case selected for full CRF based on
Mo.
Day
Year
sampling frame 1:10?
1
Yes (Complete CRF)
2
No (STOP data abstraction)
12a. ETHNIC ORIGIN:
12c. WEIGHT:
13. At time of first positive
15. Where was the patient located on the
1
Unknown
culture, patient was:
4th calendar day prior to the date of initial culture?
1
Hispanic
or Latino
_______
lbs
_______
oz OR
_______
kg
1
Pregnant
Private Residence
1
2
Not Hispanic
or Latino
1
Long Term Care Facility
2
Post-partum
12d. HEIGHT:
1
Unknown
9
Unknown
1
Long Term Acute Care Hospital
3
Neither
Homeless
1
12b. RACE:
_______
ft
_______ in OR
_______
cm
(Check all that apply)
9
Unknown
1
1
Incarcerated
White
12e. BMI:
1
1
Hospital Inpatient
Black or
Unknown
14. If case is 12 months of age,
1
African American
type of birth hospitalization:
Other
__________________________
1
American Indian
_______
1
(do not calculate, only if available in the MR)
1
NICU/SCN
or Alaska Native
Unknown
1
2
1
Asian
Well Baby Nursery
9
Native Hawaiian
Unknown
1
or Other Pacific Islander
1
Unknown
16. LOCATION OF CULTURE COLLECTION: (Check one)
17. Were cultures of the SAME or OTHER sterile site(s) positive within 30 days after initial culture date?
Hospital Inpatient
Outpatient
1
Yes
2
No
9
Unknown
5
LTCF
8
Clinic/
1
ICU
LTACH
13
If yes, indicate site and date of last positive culture:
Doctors Office
1
Internal body site
6
Surgery/OR
Autopsy
14
11
Surgery
1
Blood, Date:
________
Date:
________
1
Pericardial fluid , Date:
________
7
Radiology
Unknown
9
Dialysis/Renal Clinic
15
1
Other sterile site
Joint/Synovial fluid, Date:
2
1
CSF , Date:
________
1
________
Other Unit
Other
10
Other
(specify)
____________
4
1
Bone , Date:
________
1
Pleural fluid , Date:
________
Outpatient
Date:
________
3
Emergency Room
1
Peritoneal fluid, Date:
________
1
Muscle, Date:
______
16
Observational Unit/Clinical Decision Unit
18. PATIENT OUTCOME:
9
Unknown
1
Survived
2
Died
Mo.
Day
Year
Mo.
Day
Year
Date of death
Date of discharge
Was MRSA cultured from a normally sterile site < calendar day 7 before death?
1
Yes 2
No
If survived, was the patient transferred to a LTCF?
1
Yes 2
No 9
Unknown
If survived, was the patient transferred to a LTACH?
1
Yes 2
No
Public reporting burden of this collection of information is estimated to average 10 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of
information unless it displays a currently valid OMBcontrol number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for
reducing this burden to CDC/ATSDR Reports Clearance Officer; 1600 Clifton Road NE, MS E-11, Atlanta, Georgia 30329; ATTN: PRA (0920-0978)
Page 1 of 2
–
IMPORTANT – PLEASE COMPLETE THE BACK OF THIS FORM
–
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Legal
 1
1 2
2