Saponification Lab Page 4
ADVERTISEMENT
Name:________________________________
Date:___________________ Block:_______
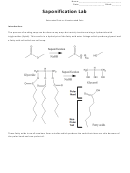
3. Measure out the total number of grams of NaOH and place into the same 400mL beaker.
(WARNING: Solid NaOH is highly basic. Use caution and wear gloves. Watch out for the residue it
leaves. )
4. Stir solution until all NaOH is dissolved. (WARNING: As solid NaOH dissolves it releases energy
which can heat up the solution.)
5. Measure out your ratio of oils and place into a separate 400mL beaker.
6. Begin heating the beaker with the oil solution in it. Keep it within the range of 37°C to 43°C
(100°F to 110°F).
7. Once the oil is within the targeted range, add the dissolved NaOH solution to the oil and begin
stirring! (If NaOH has become room temperature, carefully heat the solution back to 37°C.)
8. Take turns stirring and heating. Both must be done simultaneously for 30 minutes for
saponification to properly occur. (WARNING: Do not leave Bunsen burner under the stand while
heating. The oil will heat up really fast so remove from heat before it reaches 37°C.)
9. After 30 minutes, the soap should be thick. Let it cool to below 37°C before stirring in 3 to 5
drops of desired scented oils.
10. Pour into molds and wait 2 to 4 weeks to cure.
Post-Lab
11. After soap has cured, test it out by washing your hands. If you are worried that the soap did not
properly cure then you may wear gloves as you test it out.
Questions:
1. Did you use more saturated, unsaturated fat or equal amounts?
2. Did your soap properly cure? (How tough is it? How does it compare to a store bought bar of
soap?)
3. Did your soap lather up properly? (Was it too greasy? How does it compare to a store bought
bar of soap?)
4. If you could do this lab again, what new ratio would you try out to improve your bar of soap?
Would you use something other than Lard and Olive Oil? Explain.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4