Saponification Lab Page 2
ADVERTISEMENT
Name:________________________________
Date:___________________ Block:_______
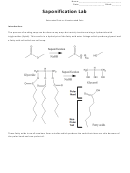
However, certain types of fats contribute specific properties in soap making. For example, most
Saturated Fats are considered unhealthy and are solid at room temperature giving it a good structure
but not a good lather. Unsaturated fats tend to produce a good amount of suds but can be too oily and
make soft bars of soap that fall apart.
Objective:
Make a bar of soap that produces suds (micelles) and has a good structure (not soft).
Pre-Lab:
Use the following charts to plan your combination of oils and fats that you would like to use to make
your soap. You can make a bar of soap that is made of just fat (or oil) if you wish, just be sure to adjust
your ratios accordingly.
Example: Let’s say you want to make a 50g bar of soap made from lard with an excess fat of 5%.
Intersecting the Lard row with the 5% column, you find the number 0.132. Multiply the 50g by 0.132
which will give you 6.6g of NaOH you will need to measure out. To figure out how much water to
dissolve the NaOH in, multiply 50g by 0.38 which will be 19g of water.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4