Density And Conservation Laws Worksheet
ADVERTISEMENT
Chemistry 511
Lab Report – Submit electronically (digital drop box) by October 9.
Note: When submitting to digital Drop Box label your files with your name first and
then a brief description of what the document is. Please note that some questions have
been changed from the original posting prior to class.
Name:
Experiment – Density and Conservation Laws
Density = Mass/Volume
DATA/CALCULATIONS
Note: Data and Calculations may be completed on an excel spreadsheet.
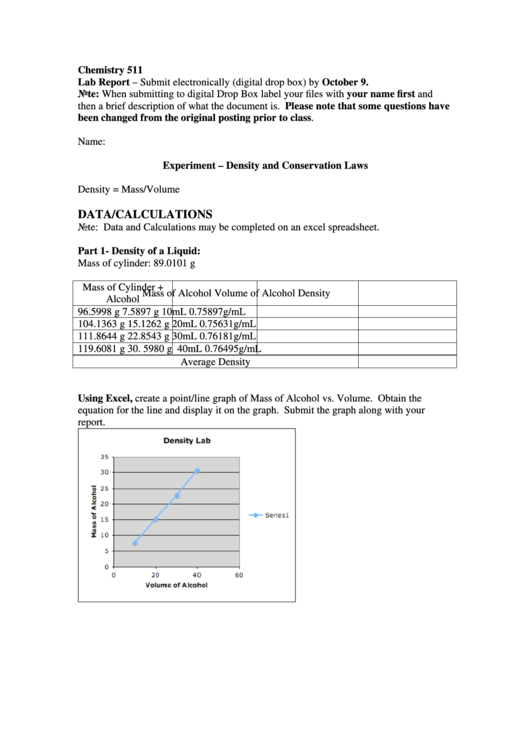
Part 1- Density of a Liquid:
Mass of cylinder: 89.0101 g
Mass of Cylinder +
Mass of Alcohol
Volume of Alcohol
Density
Alcohol
96.5998 g
7.5897 g
10mL
0.75897g/mL
104.1363 g
15.1262 g
20mL
0.75631g/mL
111.8644 g
22.8543 g
30mL
0.76181g/mL
119.6081 g
30. 5980 g
40mL
0.76495g/mL
Average Density
Using Excel, create a point/line graph of Mass of Alcohol vs. Volume. Obtain the
equation for the line and display it on the graph. Submit the graph along with your
report.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








