Flow Chart For Naming Simple Inorganic Compounds
ADVERTISEMENT
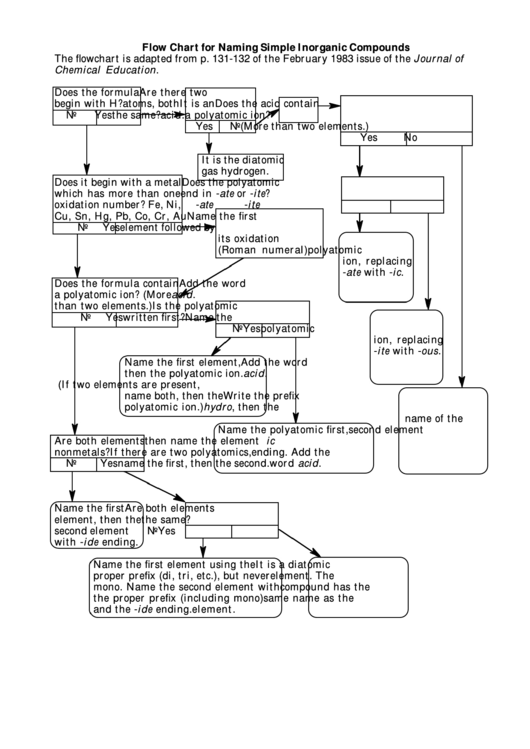
Flow Chart for Naming Simple Inorganic Compounds
The flowchart is adapted from p. 131-132 of the February 1983 issue of the Journal of
Chemical Education.
Does the formula
Are there two
begin with H?
atoms, both
It is an
Does the acid contain
No
Yes
the same?
acid.
a polyatomic ion?
Yes
No
(More than two elements.)
Yes
No
It is the diatomic
gas hydrogen.
Does it begin with a metal
Does the polyatomic
which has more than one
end in -ate or -ite?
oxidation number? Fe, Ni,
-ate
-ite
Cu, Sn, Hg, Pb, Co, Cr, Au
Name the first
No
Yes
element followed by
its oxidation number.
Name the
(Roman numeral)
polyatomic
ion, replacing
-ate with -ic.
Does the formula contain
Add the word
a polyatomic ion? (More
acid.
than two elements.)
Is the polyatomic
No
Yes
written first?
Name the
No
Yes
polyatomic
ion, replacing
-ite with -ous.
Name the first element,
Add the word
then the polyatomic ion.
acid.
(If two elements are present,
name both, then the
Write the prefix
polyatomic ion.)
hydro, then the
name of the
Name the polyatomic first,
second element
Are both elements
then name the element second.
with the -ic
nonmetals?
If there are two polyatomics,
ending. Add the
No
Yes
name the first, then the second.
word acid.
Name the first
Are both elements
element, then the
the same?
second element
No
Yes
with -ide ending.
Name the first element using the
It is a diatomic
proper prefix (di, tri, etc.), but never
element. The
mono. Name the second element with
compound has the
the proper prefix (including mono)
same name as the
and the -ide ending.
element.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1