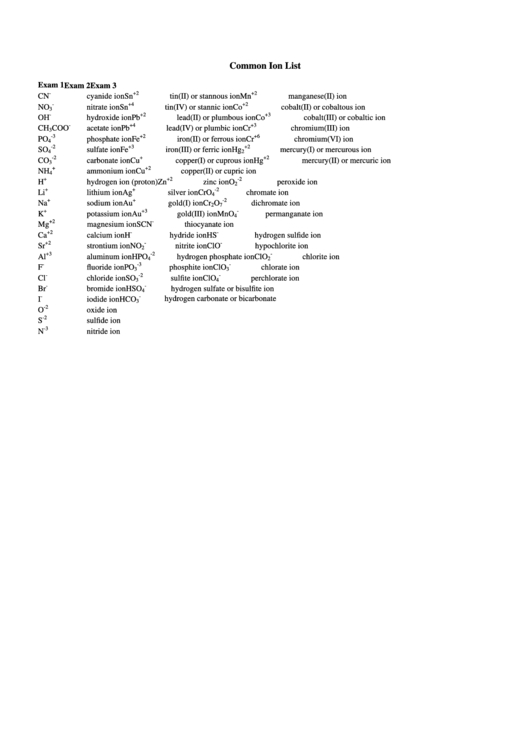
Common Ion List
ADVERTISEMENT
Common Ion List
Exam 1
Exam 2
Exam 3
-
+2
+2
CN
cyanide ion
Sn
tin(II) or stannous ion
Mn
manganese(II) ion
-
+4
+2
NO
nitrate ion
Sn
tin(IV) or stannic ion
Co
cobalt(II) or cobaltous ion
3
-
+2
+3
OH
hydroxide ion
Pb
lead(II) or plumbous ion
Co
cobalt(III) or cobaltic ion
-
+4
+3
CH
COO
acetate ion
Pb
lead(IV) or plumbic ion
Cr
chromium(III) ion
3
-3
+2
+6
PO
phosphate ion
Fe
iron(II) or ferrous ion
Cr
chromium(VI) ion
4
-2
+3
+2
SO
sulfate ion
Fe
iron(III) or ferric ion
Hg
mercury(I) or mercurous ion
4
2
-2
+
+2
CO
carbonate ion
Cu
copper(I) or cuprous ion
Hg
mercury(II) or mercuric ion
3
+
+2
NH
ammonium ion
Cu
copper(II) or cupric ion
4
+
+2
-2
H
hydrogen ion (proton)
Zn
zinc ion
O
peroxide ion
2
+
+
-2
Li
lithium ion
Ag
silver ion
CrO
chromate ion
4
+
+
-2
Na
sodium ion
Au
gold(I) ion
Cr
O
dichromate ion
2
7
+
+3
-
K
potassium ion
Au
gold(III) ion
MnO
permanganate ion
4
+2
-
Mg
magnesium ion
SCN
thiocyanate ion
+2
-
-
Ca
calcium ion
H
hydride ion
HS
hydrogen sulfide ion
+2
-
-
Sr
strontium ion
NO
nitrite ion
ClO
hypochlorite ion
2
+3
-2
-
Al
aluminum ion
HPO
hydrogen phosphate ion
ClO
chlorite ion
4
2
-
-3
-
F
fluoride ion
PO
phosphite ion
ClO
chlorate ion
3
3
-
-2
-
Cl
chloride ion
SO
sulfite ion
ClO
perchlorate ion
3
4
-
-
Br
bromide ion
HSO
hydrogen sulfate or bisulfite ion
4
-
-
hydrogen carbonate or bicarbonate
I
iodide ion
HCO
3
-2
O
oxide ion
-2
S
sulfide ion
-3
N
nitride ion
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3








